Protein Dynamical Transition
|
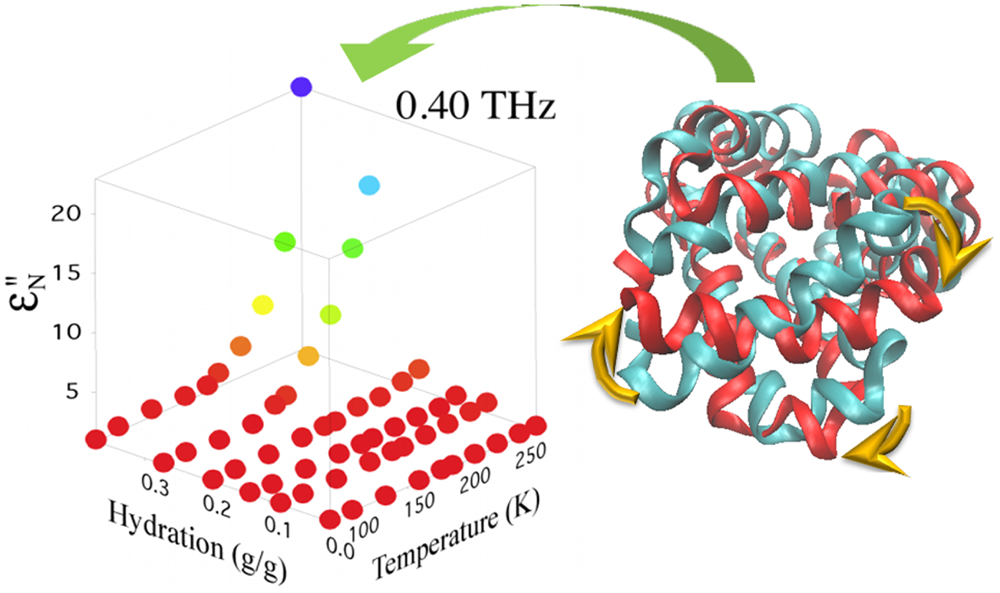
Protein dynamics are intertwined with the solvent dynamics. A minimum hydration is necessary for proteins to function. The nature of the protein-solvent interaction has been exemplified by the observation of a turn on in protein dynamics at a temperature that appears largely dictated by the hydration. This turn on can be characterized using terahertz absorption measurements of solutions. Using THz time domain spectroscopy we explore how the protein-solvent interactions change with protein conformation and functional state. For example we have found that the turn on temperature shifts with inhibitor binding for enzymes and with photo bleaching for fluorescent proteins. We examine how these shifts may provide a characterization of the average protein-solvent interaction energy. |
|
(After [2]) |
Return to Research |